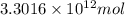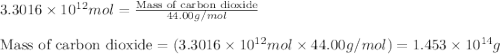Chemistry, 17.09.2019 20:10 samanthasheets8006
Assuming gasoline is 89.0% isooctane, with a density of 0.692 g/ml, what is the theoretical yield (in grams) of co2 produced by the combustion of 1.80 x 1010 gallons of gasoline (the estimated annual consumption of gasoline in the u. remember, there are 3.785 liters in 1 gallon and assume that isooctane is the only carbon containing component of gasoline. scientific notation can be entered as follows: 1.23 x 1023 = 1.23e23

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Assuming gasoline is 89.0% isooctane, with a density of 0.692 g/ml, what is the theoretical yield (i...
Questions


Biology, 23.03.2020 17:23




Mathematics, 23.03.2020 17:23






Mathematics, 23.03.2020 17:23




World Languages, 23.03.2020 17:23




Mathematics, 23.03.2020 17:23

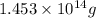
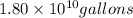
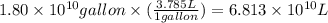
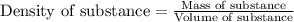
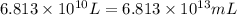 (Conversion factor: 1 L = 1000 mL)
(Conversion factor: 1 L = 1000 mL)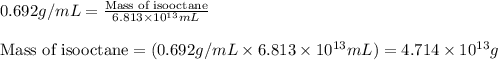
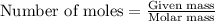 .....(1)
.....(1)
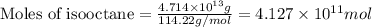
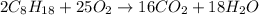
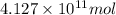 of isooctane will produce =
of isooctane will produce = 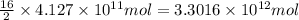 of carbon dioxide
of carbon dioxide