
Based on their activation energies and energy changes and assuming that all collision factors are the same, which of the following reactions would be fastest? (a) ea = 57 kj/mol , δe = 13 kj/mol (b) ea = 47 kj/mol , δe = -29 kj/mol (c) ea = 34 kj/mol , δe = -10 kj/mol .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1


Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
You know the right answer?
Based on their activation energies and energy changes and assuming that all collision factors are th...
Questions

History, 28.01.2021 23:40



History, 28.01.2021 23:40

Mathematics, 28.01.2021 23:40

Health, 28.01.2021 23:40


Arts, 28.01.2021 23:40

Mathematics, 28.01.2021 23:40

Mathematics, 28.01.2021 23:40

Mathematics, 28.01.2021 23:40

Social Studies, 28.01.2021 23:40


Mathematics, 28.01.2021 23:40

Mathematics, 28.01.2021 23:40

Biology, 28.01.2021 23:40

Mathematics, 28.01.2021 23:40

Chemistry, 28.01.2021 23:40

English, 28.01.2021 23:40

Mathematics, 28.01.2021 23:40

![r=k.[A]^{n}](/tpl/images/0237/1684/d8a47.png) [1]
[1]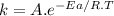 [2]
[2]

