
Iron reacts with oxygen to produce iron(iii) oxide as represented above. a 75.0 g sample of fe(s)is mixed with 11.5 l of o2(g) at 2.66 atm and 298 k.(a) calculate the number of moles of each of the following before the reaction occurs.(i) fe(s)(ii) o2(g)(b) identify the limiting reactant when the mixture is heated to produce fe2o3. support youranswer with calculations.(c) calculate the number of moles of fe2o3 produced when the reaction proceeds tocompletion.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 09:30
The allotropes of carbon include a variety of structures that include three-dimensional tetrahedral lattices, planes of hexagonal rings, cylindrical tubes of hexagonal rings, and spheres of five- and six-membered rings. similar shapes of network covalent atomic solids are possible with carbon nitride, boron, and pure silicon (e.g., silicene is a graphene-like allotrope of pure silicon). in contrast, silicates exist as either highly ordered or amorphous (more random) three-dimensional lattices. what could explain why there are there no naturally occurring sheets, stacked sheets, cylindrical tubes, or spheres of network covalent atomic solids composed of silicon and oxygen (sio2)? would pure silicate structures make good lubricants or good electrical conductors?
Answers: 3

Chemistry, 23.06.2019 14:00
How can a ringing telephone can be heard through a closed door
Answers: 1
You know the right answer?
Iron reacts with oxygen to produce iron(iii) oxide as represented above. a 75.0 g sample of fe(s)is...
Questions

Chemistry, 07.10.2021 02:40


Mathematics, 07.10.2021 02:40

Mathematics, 07.10.2021 02:40

Mathematics, 07.10.2021 02:40

Chemistry, 07.10.2021 02:40


Mathematics, 07.10.2021 02:40

Biology, 07.10.2021 02:40

French, 07.10.2021 02:40


Spanish, 07.10.2021 02:40

Mathematics, 07.10.2021 02:40

Social Studies, 07.10.2021 02:40

Health, 07.10.2021 02:40

History, 07.10.2021 02:40


Computers and Technology, 07.10.2021 02:40


Law, 07.10.2021 02:40

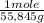 = 1,34 Fe(s) moles
= 1,34 Fe(s) moles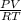 = n
= n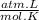
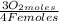 = 1,01 O₂ moles
= 1,01 O₂ moles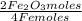 =
= 

