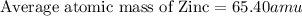2.3 zinc has five naturally occurring isotopes: 48.63% of 64 zn with an atomic weight of 63.929 amu; 27.90% of 66zn with an atomic weight of 65.926 amu; 4.10% of 67zn with an atomic weight of 66.927 amu; 18.75% of 68zn with an atomic weight of 67.925 amu; and 0.62% of 70zn with an atomic weight of 69.925 amu. calculate the average atomic weight of zn.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
2.3 zinc has five naturally occurring isotopes: 48.63% of 64 zn with an atomic weight of 63.929 amu...
Questions

Mathematics, 15.07.2020 09:01

Social Studies, 15.07.2020 09:01

Mathematics, 15.07.2020 09:01



Spanish, 15.07.2020 09:01

Mathematics, 15.07.2020 09:01

English, 15.07.2020 09:01




Mathematics, 15.07.2020 09:01


History, 15.07.2020 09:01

Mathematics, 15.07.2020 09:01

Physics, 15.07.2020 09:01

Mathematics, 15.07.2020 09:01

Mathematics, 15.07.2020 09:01

Mathematics, 15.07.2020 09:01

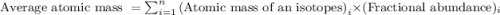 .....(1)
.....(1)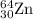 isotope:
isotope: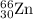 isotope:
isotope: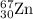 isotope:
isotope: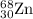 isotope:
isotope: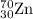 isotope:
isotope:![\text{Average atomic mass of Zinc}=[(63.929\times 0.4863)+(65.926\times 0.2790)+(66.927\times 0.0410)+(67.925\times 0.1875)+(69.925\times 0.0062)]](/tpl/images/0237/0119/27e73.png)