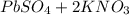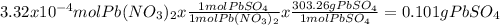Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
When aqueous solutions of k₂so₄ and pb(no₃)₂ are combined, pbso₄ precipitates. calculate the mass, i...
Questions



Mathematics, 22.04.2021 16:20

Chemistry, 22.04.2021 16:20

History, 22.04.2021 16:20

Chemistry, 22.04.2021 16:20

Mathematics, 22.04.2021 16:20



Physics, 22.04.2021 16:20


Business, 22.04.2021 16:20



Mathematics, 22.04.2021 16:20

History, 22.04.2021 16:20



History, 22.04.2021 16:20

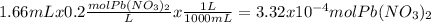
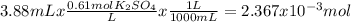
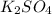
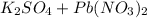 ⇒
⇒