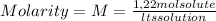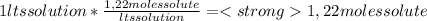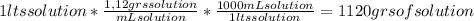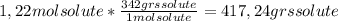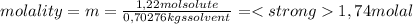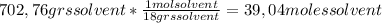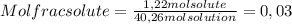Chemistry, 17.09.2019 02:00 romaguera06
A) calculatethe molality, m, of an aqueous solution of 1.22 m sucrose, c12h22o11. the density of the solution is 1.12 g/ml. b) what is the mass percent of sucrose in this solution? c) what is the mole fraction of sucrose in this solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2
You know the right answer?
A) calculatethe molality, m, of an aqueous solution of 1.22 m sucrose, c12h22o11. the density of the...
Questions


Geography, 18.09.2019 14:00

History, 18.09.2019 14:00






Business, 18.09.2019 14:00

English, 18.09.2019 14:00


Physics, 18.09.2019 14:00


Geography, 18.09.2019 14:00

Chemistry, 18.09.2019 14:00

Mathematics, 18.09.2019 14:00

Mathematics, 18.09.2019 14:00



Mathematics, 18.09.2019 14:00