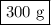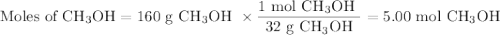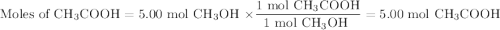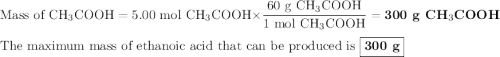The following reaction produces ethanoic acid (chacooh) from methanol (ch3oh) and carbon
monox...

Chemistry, 17.09.2019 01:00 ayeelol1447
The following reaction produces ethanoic acid (chacooh) from methanol (ch3oh) and carbon
monoxide
chcooh
ch3oh + co
relative atomie mass: h = 1: 0 = 16; c = 12
calculate the maximum mass of ethanoic acid that can be produced from 160g methanol,
assuming the carbon monoxide is in excess.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2


Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
Questions


Social Studies, 12.01.2021 21:30

Computers and Technology, 12.01.2021 21:30



Arts, 12.01.2021 21:30

History, 12.01.2021 21:30

Arts, 12.01.2021 21:30


Mathematics, 12.01.2021 21:30


Chemistry, 12.01.2021 21:30




Mathematics, 12.01.2021 21:30


English, 12.01.2021 21:30


Mathematics, 12.01.2021 21:30