
Chemistry, 16.09.2019 22:00 ericahale3971
Suppose 6.54g of potassium bromide is dissolved in 50.ml of a 0.70 m aqueous solution of silver nitrate. calculate the final molarity of bromide anion in the solution. you can assume the volume of the solution doesn't change when the potassium bromide is dissolved in it.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
Suppose 6.54g of potassium bromide is dissolved in 50.ml of a 0.70 m aqueous solution of silver nitr...
Questions


Health, 17.12.2020 05:10

Business, 17.12.2020 05:10

English, 17.12.2020 05:10


Mathematics, 17.12.2020 05:10

English, 17.12.2020 05:10

Social Studies, 17.12.2020 05:10



Arts, 17.12.2020 05:10


Chemistry, 17.12.2020 05:10

Health, 17.12.2020 05:10

Chemistry, 17.12.2020 05:10

Mathematics, 17.12.2020 05:10




Arts, 17.12.2020 05:10

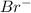 ]=0.4M
]=0.4M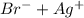 ⇄
⇄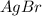 Kps for this reaction is
Kps for this reaction is 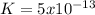 , this constant indicates if the reaction can happen spontaneously or not. in this case, a small value of Kps means that precipitate can be formed.
, this constant indicates if the reaction can happen spontaneously or not. in this case, a small value of Kps means that precipitate can be formed.![[Br^{-}]=6.54gKBr.\frac{1molKBr}{119gKBr}.\frac{1molBr^{-} }{1molKBr} .\frac{1}{0.05L} =1.10M](/tpl/images/0234/3240/bfec1.png)
![Qps=[Br^{-}][Ag^{+}]](/tpl/images/0234/3240/78356.png) =
=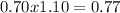
![[Br^{-}]=\frac{(\frac{1.1mol}{L}.0.05L-\frac{0.70mol}{L}.0.05L}{0.05L} =0.4M](/tpl/images/0234/3240/ff056.png)


