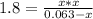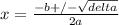Chemistry, 16.09.2019 19:30 gracebuffum
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(g)kc=1.80 at 250 ∘c a 0.157 mol sample of pcl5(g) is injected into an empty 2.50 l reaction vessel held at 250 ∘c. calculate the concentrations of pcl5(g) and pcl3(g) at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(g)kc=1...
Questions

Mathematics, 02.09.2021 23:00

Mathematics, 02.09.2021 23:00

Mathematics, 02.09.2021 23:00

Mathematics, 02.09.2021 23:00



Social Studies, 02.09.2021 23:00





Mathematics, 02.09.2021 23:00

English, 02.09.2021 23:00

English, 02.09.2021 23:00





Mathematics, 02.09.2021 23:00

Mathematics, 02.09.2021 23:00

![Kc = \frac{[C]^cx[D]^d}{[A]^ax[B]^b}](/tpl/images/0233/9746/d3f86.png)
![Kc = \frac{[PCl3]x[Cl2]}{[PCl5]}](/tpl/images/0233/9746/20f28.png)