

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

You know the right answer?
Calculate the atomic weight of rubidium, which has two isotopes with the following properties: 85^r...
Questions

Mathematics, 13.06.2021 21:20


Mathematics, 13.06.2021 21:20



Mathematics, 13.06.2021 21:20

Mathematics, 13.06.2021 21:20


Medicine, 13.06.2021 21:20

Social Studies, 13.06.2021 21:20





Mathematics, 13.06.2021 21:20



Mathematics, 13.06.2021 21:20


Mathematics, 13.06.2021 21:20

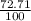 = 61.73 amu
= 61.73 amu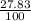 = 24.18 amu
= 24.18 amu

