
Phosphorus forms several compounds with oxygen, including diphosphorus trioxide and diphosphorus pentoxide. a decomposition of a sample of diphosphorus trioxide forms 1.29 g phosphorus to every 1.00 g oxygen. the decomposition of a sample of diphosphorus pentoxide forms 0.775 g phosphorus to every 1.00 g oxygen. show that these results are consistent with the law of multiple proportions.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1


Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
Phosphorus forms several compounds with oxygen, including diphosphorus trioxide and diphosphorus pen...
Questions

Chemistry, 04.11.2020 07:20

Mathematics, 04.11.2020 07:20


Mathematics, 04.11.2020 07:20




Mathematics, 04.11.2020 07:20

Mathematics, 04.11.2020 07:20

History, 04.11.2020 07:20



Mathematics, 04.11.2020 07:20





Mathematics, 04.11.2020 07:20

Physics, 04.11.2020 07:20

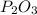 the ratio between P and O is
the ratio between P and O is 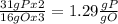 , that is consistent with the experimental result.
, that is consistent with the experimental result.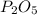 the ratio between P and O is
the ratio between P and O is 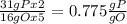 that is also consistent with experimental results. In conclusion we can say that the results obtained are consistent with the law of multiple proportions.
that is also consistent with experimental results. In conclusion we can say that the results obtained are consistent with the law of multiple proportions.

