
Chemistry, 16.09.2019 17:10 briweaver9993
Titanium dioxide, tio₂, reacts with carbon and chlorine to give gaseous ticl₄: tio₂+2c+2ci₂−tici₄+2co the reaction of 7.39 kg titanium dioxide with excess c and cl₂ gives 14.24 kg titanium tetrachloride. calculate the theoretical yield of ticl₄ (assuming complete reaction) and its percentage yield.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Titanium dioxide, tio₂, reacts with carbon and chlorine to give gaseous ticl₄: tio₂+2c+2ci₂−tici₄+2...
Questions


Health, 12.10.2019 08:50


Biology, 12.10.2019 08:50


Biology, 12.10.2019 08:50

Mathematics, 12.10.2019 08:50

Mathematics, 12.10.2019 08:50


Health, 12.10.2019 08:50

History, 12.10.2019 08:50


Mathematics, 12.10.2019 08:50



Spanish, 12.10.2019 08:50

Mathematics, 12.10.2019 08:50

Social Studies, 12.10.2019 08:50


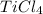 and its percentage yield is 81.0%
and its percentage yield is 81.0%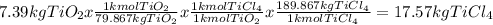
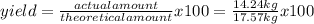 =81.0%
=81.0%

