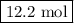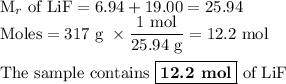Chemistry, 14.09.2019 11:30 TyleenValdez975
Calculating and using the molar mass of heterodiatomic the chemical formula for lithium fluoride is lif a chemist measured the amount of lithium fluoride produced during an experiment. she finds that 317. g of lithium fluoride is produced. calculate the number of moles of lithium fluoride produced. round your answer to 3 significant digits. mol x explanation check 2019 mcgrawh terms of education all righes reserved

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1


Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Calculating and using the molar mass of heterodiatomic the chemical formula for lithium fluoride is...
Questions

Social Studies, 06.04.2020 19:03




Mathematics, 06.04.2020 19:03

English, 06.04.2020 19:03

History, 06.04.2020 19:03





Mathematics, 06.04.2020 19:03



Mathematics, 06.04.2020 19:03

Biology, 06.04.2020 19:03

Mathematics, 06.04.2020 19:03


Mathematics, 06.04.2020 19:03

Mathematics, 06.04.2020 19:03