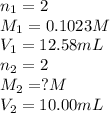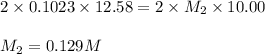Chemistry, 14.09.2019 11:30 chloehall2269
Ranjit titrates a sample 10.00 ml of ba(oh)2 solution to the endpoint using 12.58 ml of 0.1023 m h2so4.
based on this data, calculate the concentration of the barium hydroxide solution.
[ba(oh)2] = m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1


Chemistry, 23.06.2019 13:30
Use the periodic table to classify each of the elements below. cadmium (cd): vanadium (v): xenon (xe): iodine (i): potassium (k): strontium (sr):
Answers: 3
You know the right answer?
Ranjit titrates a sample 10.00 ml of ba(oh)2 solution to the endpoint using 12.58 ml of 0.1023 m h2s...
Questions



Physics, 18.10.2020 14:01




History, 18.10.2020 14:01

History, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01



Mathematics, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01


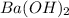 comes out to be 0.129 M.
comes out to be 0.129 M.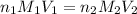
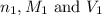 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 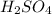
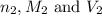 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is