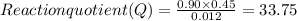Chemistry, 14.09.2019 11:30 tanyadeewill
7. for the system pcls(g) → pc13(g) + cl2(g) kis 26 at 300°c. in a 5.0-l flask, a gaseous mixture consists of all three gases with partial pressure as follows: ppcis = 0.012 atm, pc2=0.45 atm, ppci3 -0.90 atm. a) is the mixture at equilibrium? explain. b) if it is not at equilibrium, which way will the system shift to establish equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
7. for the system pcls(g) → pc13(g) + cl2(g) kis 26 at 300°c. in a 5.0-l flask, a gaseous mixture co...
Questions


Mathematics, 01.08.2019 19:50

History, 01.08.2019 19:50



Mathematics, 01.08.2019 19:50


Mathematics, 01.08.2019 19:50


Geography, 01.08.2019 19:50


Mathematics, 01.08.2019 19:50




Mathematics, 01.08.2019 19:50


Social Studies, 01.08.2019 19:50

Mathematics, 01.08.2019 19:50

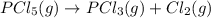
![Reaction\ quotient (Q) = \frac{[p_{PCl_3}]\times [p_{Cl_2}]}{[p_{PCl_5}]}](/tpl/images/0231/3431/4e28d.png)
![[p_{PCl_5}] = 0.012 atm](/tpl/images/0231/3431/6af84.png)
![[p_{PCl_3}]= 0.90 atm](/tpl/images/0231/3431/1d822.png)
![[p_{Cl_2}]= 0.45 atm](/tpl/images/0231/3431/b9835.png)