
You want to determine the density of a compound but have only tiny crystal, and it would be difficult to measure mass and volume accurately. there is another way to determine density, however called the flotation method. if you placed the crystal in a liquid whose density is precisely that of the substance, it would be suspended in the liquid, neither sinking to the bottom of the beaker nor floating to the surface. however, for such an experiment, you would need to have a liquid with the precise density of the crystal. you can accomplish this by mixing two liquids of different densities to create a liquid having the desired density a a consider the following: you mix 7.30 ml of chci3 (d = 1.492 g/ml) and 8.90 ml of chbt3 (d = 2.890 g/ml) giving 16.2 ml of solution. what is the density of this mixture? density = g/ml

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which features are shown in the image? check all that apply. folds o anticlines o synclines o normal faults ostrike-slip faults
Answers: 1


Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
You want to determine the density of a compound but have only tiny crystal, and it would be difficul...
Questions








Mathematics, 24.09.2019 03:10








Mathematics, 24.09.2019 03:10

Mathematics, 24.09.2019 03:10

Health, 24.09.2019 03:10



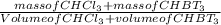
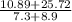 = 2.26g/mL
= 2.26g/mL

