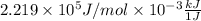Chemistry, 14.09.2019 09:30 victoriadorvilu
The vapor pressure of substance x is 100. mm hg at 1080.°c. the vapor pressure of substance x increases to 600. mm hg at 1220.°c. determine the molar heat of vaporization of substance x using the derived form of the clausius-clapeyron equation given below. (include the sign of the value in your answer.) kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
The vapor pressure of substance x is 100. mm hg at 1080.°c. the vapor pressure of substance x increa...
Questions

Chemistry, 12.07.2019 00:40

Mathematics, 12.07.2019 00:40

Mathematics, 12.07.2019 00:40


Mathematics, 12.07.2019 00:40

Social Studies, 12.07.2019 00:40

Business, 12.07.2019 00:40

Social Studies, 12.07.2019 00:40

Chemistry, 12.07.2019 00:40

Biology, 12.07.2019 00:40

Biology, 12.07.2019 00:40

Biology, 12.07.2019 00:40

Biology, 12.07.2019 00:40

Biology, 12.07.2019 00:40

Mathematics, 12.07.2019 00:40

English, 12.07.2019 00:40

History, 12.07.2019 00:40


Mathematics, 12.07.2019 00:40

Mathematics, 12.07.2019 00:40

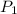 = 100 mm Hg or
= 100 mm Hg or 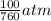 = 0.13157 atm
= 0.13157 atm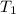 =
= 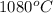 = (1080 + 273) K = 1357 K
= (1080 + 273) K = 1357 K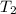 =
= 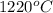 = (1220 + 273) K = 1493 K
= (1220 + 273) K = 1493 K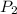 = 600 mm Hg or
= 600 mm Hg or 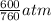 = 0.7895 atm
= 0.7895 atm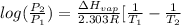
![log(\frac{0.7895}{0.13157}) = \frac{\Delta H_{vap}}{2.303 \times 8.314 J/mol K}[\frac{1}{1357 K} - \frac{1}{1493 K}]](/tpl/images/0231/2242/21278.png)
![log (6) = \frac{\Delta H_{vap}}{19.147}[\frac{(1493 - 1357) K}{1493 K \times 1357 K}]](/tpl/images/0231/2242/46d38.png)
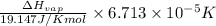
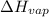 =
= 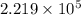 J/mol
J/mol