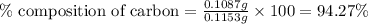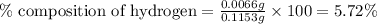Chemistry, 14.09.2019 09:30 bagofmud8339
A0.1153-gram sample of a pure hydrocarbon was burned in a c-h combustion train to produce 0.3986 gram of co2and 0.0578 gram of h2o. determine the masses of c and h in the sample and the percentages of these elements in this hydrocarbon.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3


You know the right answer?
A0.1153-gram sample of a pure hydrocarbon was burned in a c-h combustion train to produce 0.3986 gra...
Questions



Social Studies, 12.08.2019 18:10
















Computers and Technology, 12.08.2019 18:10

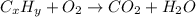
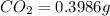
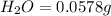
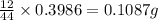 of carbon will be contained.
of carbon will be contained.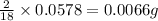 of hydrogen will be contained.
of hydrogen will be contained.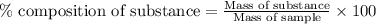 ......(1)
......(1)