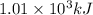Chemistry, 14.09.2019 09:30 litwork5885
Hexane is a common laboratory solvent and a component of petrol. its combustion reaction is as follows: 2 c6h14 (9) + 19 02(9) +12 co2(g) + 14 h20(1) arh=-8326 kj mol-1 use the reaction enthalpy value to calculate the heat in (kj) released by combustion of 0.121 mol of hexane. (give your answer as a positive number to at least three significant figures and do not include the units in the answer box)

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 11:30
If this sedimentary rock layer is truly the oldest one of marine origin, what do you think that tells usabout the formation of earth's oceans?
Answers: 2
You know the right answer?
Hexane is a common laboratory solvent and a component of petrol. its combustion reaction is as follo...
Questions





Computers and Technology, 28.01.2020 00:31


Mathematics, 28.01.2020 00:31


Mathematics, 28.01.2020 00:31

Mathematics, 28.01.2020 00:31

Mathematics, 28.01.2020 00:31

Mathematics, 28.01.2020 00:31


Advanced Placement (AP), 28.01.2020 00:31

Social Studies, 28.01.2020 00:31


Social Studies, 28.01.2020 00:31



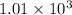
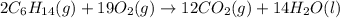
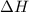 = -8326 kJ/mol
= -8326 kJ/mol