
Chemistry, 14.09.2019 09:30 babygurl27732
If 1.0 m hcl is added to an equal volume of 0.2 m naoh, what are the new concentrations of oh and h30+ ? select one: [h30*] = 0.8 m, [oh]= 1.3 x 10-14 m h30*] = 0.5 m, [oh]= 0.1 mi h30*] = 0.4 m, [oh]= 2.5 x 1014 m h30*] 1.0 m, [0h]= 0.2 m h30*] = 0.5 m, [oh]= 2.0 x 1014 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

You know the right answer?
If 1.0 m hcl is added to an equal volume of 0.2 m naoh, what are the new concentrations of oh and h3...
Questions

Mathematics, 21.05.2021 17:30

French, 21.05.2021 17:30




Mathematics, 21.05.2021 17:30



Mathematics, 21.05.2021 17:30

Mathematics, 21.05.2021 17:30

History, 21.05.2021 17:30






Chemistry, 21.05.2021 17:30

History, 21.05.2021 17:30


SAT, 21.05.2021 17:30

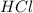 is a strong acid and
is a strong acid and 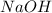 is a strong base. The resulting reaction is a neutralization reaction forming
is a strong base. The resulting reaction is a neutralization reaction forming 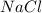 and
and 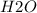

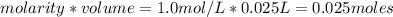
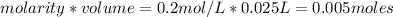
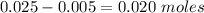
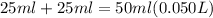
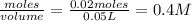
![[H3O+][OH-] = 10^{-14}\\ \\[OH-] = \frac{10^{-14} }{[H3O+]} = \frac{10^{-14} }{0.4} =2.5*10^{-14}](/tpl/images/0231/2255/824f2.png)


