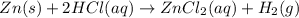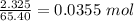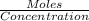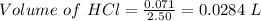Chemistry, 14.09.2019 09:20 miriamnelson7545
Zinc reacts with hydrochloric acid according to the reaction equation shown. zn(s) + 2 hcl(aq) → zncl2(aq) + h2(g) how many milliliters of 2.50 m hcl(aq) are required to react with 4.65 g of an ore containing 50.0% zn(s) by mass? volume: ml

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
Zinc reacts with hydrochloric acid according to the reaction equation shown. zn(s) + 2 hcl(aq) → znc...
Questions

Mathematics, 12.03.2021 19:00

Mathematics, 12.03.2021 19:00


English, 12.03.2021 19:00


English, 12.03.2021 19:00

Mathematics, 12.03.2021 19:00

Mathematics, 12.03.2021 19:00


Mathematics, 12.03.2021 19:00


Mathematics, 12.03.2021 19:00



Mathematics, 12.03.2021 19:00


History, 12.03.2021 19:00

Mathematics, 12.03.2021 19:00

Mathematics, 12.03.2021 19:00

Arts, 12.03.2021 19:00