
Chemistry, 14.09.2019 08:30 brianamitzel1013
500 ml of a solution contains 1000 mg of cacl2. molecular weight of cacl2 is 110 g/mol. specific gravity of the solution is 0. cacl2 = ca++ + 2cl-
a) express the concentration of the solution in % w/v
b) express the concentration in ratio strength
c) express the concentration in molarity (m)
d) express the concentration in molality (m)
e) how many equivalents of calcium chloride would be in 1.5 l of the solution?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2


Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
500 ml of a solution contains 1000 mg of cacl2. molecular weight of cacl2 is 110 g/mol. specific gra...
Questions



Mathematics, 26.02.2021 21:40



Mathematics, 26.02.2021 21:40

Mathematics, 26.02.2021 21:40



Mathematics, 26.02.2021 21:40

Mathematics, 26.02.2021 21:40



Chemistry, 26.02.2021 21:40


Mathematics, 26.02.2021 21:40




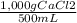 ×100 = 0,2%w/v
×100 = 0,2%w/v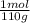 = 9,09×10⁻³ moles of CaCl₂
= 9,09×10⁻³ moles of CaCl₂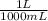 = 0,500 L of solution
= 0,500 L of solution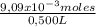 = 0,0182 M
= 0,0182 M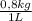 = 0,625 kg of solution
= 0,625 kg of solution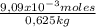 = 0,0145 m
= 0,0145 m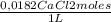 ×
× 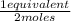 = 0,0137 equivalents
= 0,0137 equivalents


