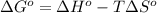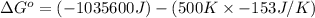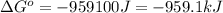Use the given data at 500 k to calculate δg°for the reaction
2h2s(g) + 3o2(g) → 2h2o(g) + 2so2...

Chemistry, 14.09.2019 08:20 87haymaker
Use the given data at 500 k to calculate δg°for the reaction
2h2s(g) + 3o2(g) → 2h2o(g) + 2so2(g)
substance h2s(g) o2(g) h2o(g) so2(g)
δh°f(kj/mol) -21 0 -242 -296.8
s°(j/k·mol) 206 205 189 248

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Questions









Mathematics, 25.02.2020 00:37



Computers and Technology, 25.02.2020 00:38


Mathematics, 25.02.2020 00:38






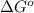 for the reaction is -959.1 kJ
for the reaction is -959.1 kJ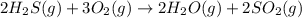
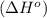 .
.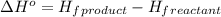
![\Delta H^o=[n_{H_2O}\times \Delta H_f^0_{(H_2O)}+n_{SO_2}\times \Delta H_f^0_{(SO_2)}]-[n_{H_2S}\times \Delta H_f^0_{(H_2S)}+n_{O_2}\times \Delta H_f^0_{(O_2)}]](/tpl/images/0231/1095/c2362.png)
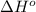 = enthalpy of reaction = ?
= enthalpy of reaction = ?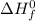 = standard enthalpy of formation
= standard enthalpy of formation![\Delta H^o=[2mole\times (-242kJ/mol)+2mole\times (-296.8kJ/mol)}]-[2mole\times (-21kJ/mol)+3mole\times (0kJ/mol)]](/tpl/images/0231/1095/0563e.png)
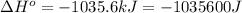
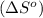 .
.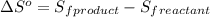
![\Delta S^o=[n_{H_2O}\times \Delta S_f^0_{(H_2O)}+n_{SO_2}\times \Delta S_f^0_{(SO_2)}]-[n_{H_2S}\times \Delta S_f^0_{(H_2S)}+n_{O_2}\times \Delta S_f^0_{(O_2)}]](/tpl/images/0231/1095/f7046.png)
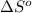 = entropy of reaction = ?
= entropy of reaction = ?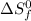 = standard entropy of formation
= standard entropy of formation![\Delta S^o=[2mole\times (189J/K.mol)+2mole\times (248J/K.mol)}]-[2mole\times (206J/K.mol)+3mole\times (205J/K.mol)]](/tpl/images/0231/1095/0797b.png)
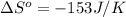
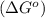 .
.