Calculate δs°for the combustion of ammonia.
4nh3(g) + 3o2(g) → 2n2(g) + 6h2o(l)
substanc...

Chemistry, 14.09.2019 08:20 serenityarts123
Calculate δs°for the combustion of ammonia.
4nh3(g) + 3o2(g) → 2n2(g) + 6h2o(l)
substance nh3(g) o2(g) n2(g) h2o(l)
s°(j/k·mol) 192 205.1 192 70
-135 j
-579 j
-387 j
579 j

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
Questions

Spanish, 17.09.2019 21:00


Mathematics, 17.09.2019 21:00






Biology, 17.09.2019 21:00

Mathematics, 17.09.2019 21:00

Geography, 17.09.2019 21:00


Mathematics, 17.09.2019 21:00

Mathematics, 17.09.2019 21:00


English, 17.09.2019 21:00

History, 17.09.2019 21:00


Mathematics, 17.09.2019 21:00

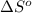 of the reaction is
of the reaction is 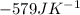
![\Delta S_{rxn}=\sum [n\times \Delta S^o_{products}]-\sum [n\times \Delta S^o_{reactants}]](/tpl/images/0231/1100/ec939.png)
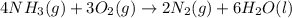
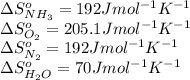
![\Delta S^o_{rxn}=[(6\times \Delta S^o_{H_2O})+(2\times \Delta S^o_{N_2})]-[(4\times \Delta S^o_{NH_3})+(3\times \Delta S^o_{O_2})]](/tpl/images/0231/1100/480ae.png)
![\Delta S^o_{rxn}=[(6\times 70)+(2\times 192)]-[(4\times 192)+(3\times 205.1)]=-579JK^{-1}](/tpl/images/0231/1100/8f98e.png)


