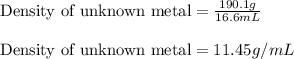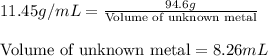Chemistry, 14.09.2019 08:10 caylah5921
Apiece of an unknown metal has a volume of 16.6 ml and a mass of 190.1 grams. the density of the metal is g/ml a piece of the same metal with a mass of 94.6 grams would have a volume of ml. submit answer

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3


Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
Apiece of an unknown metal has a volume of 16.6 ml and a mass of 190.1 grams. the density of the met...
Questions

Mathematics, 21.07.2019 05:30


History, 21.07.2019 05:30

Mathematics, 21.07.2019 05:30

Advanced Placement (AP), 21.07.2019 05:30




Mathematics, 21.07.2019 05:30

English, 21.07.2019 05:30

Mathematics, 21.07.2019 05:30



Social Studies, 21.07.2019 05:30

Spanish, 21.07.2019 05:30



Mathematics, 21.07.2019 05:30


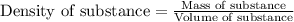 ......(1)
......(1)