
Chemistry, 14.09.2019 07:30 Bamaboy8804
The heat of vaporization of water is: o a. the amount of heat/energy required to convert 1 gram of water at 0°c to 1 gram of steam at 100°c. o b. the amount of heat/energy required to convert 1 gram of ice at 0°c to 1 gram of liquid water at 0°c. o c. the amount of heat/energy required to convert 1 gram of liquid water at 100°c to 1 gram of steam at 100°c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
The heat of vaporization of water is: o a. the amount of heat/energy required to convert 1 gram of...
Questions


History, 19.10.2021 23:40

English, 19.10.2021 23:40


Business, 19.10.2021 23:40

Mathematics, 19.10.2021 23:40



World Languages, 19.10.2021 23:40


Mathematics, 19.10.2021 23:40

Mathematics, 19.10.2021 23:40


Mathematics, 19.10.2021 23:40




Mathematics, 19.10.2021 23:40

History, 19.10.2021 23:40

Biology, 19.10.2021 23:40

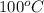 .
.

