
Chemistry, 25.12.2019 20:31 tommylopez22713
Consider the following reversible, exothermic chemical reaction: 2c2h2(g) + 5o2(g) ↔4co2(g) + 2h2o(g)
all but one of the conditions will cause equilibrium to shift right and produce more of the products. which change will cause the reaction to shift to the left?
a) adding oxygen
b) adding co2
c) increasing the pressure
d) decreasing the temperature

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
Consider the following reversible, exothermic chemical reaction: 2c2h2(g) + 5o2(g) ↔4co2(g) + 2h2o(...
Questions


Mathematics, 01.04.2020 20:54

Chemistry, 01.04.2020 20:54

History, 01.04.2020 20:54



Mathematics, 01.04.2020 20:54

Mathematics, 01.04.2020 20:54



Biology, 01.04.2020 20:54







History, 01.04.2020 20:54


Biology, 01.04.2020 20:54

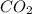
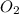 is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of
is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of 

