Prob.: consider the combustion of butane (c4h10):
2c4h10(g) + 13o2(g) ==> 8co2(g) + 10 h...

Chemistry, 14.09.2019 00:30 zabomoxx5ll
Prob.: consider the combustion of butane (c4h10):
2c4h10(g) + 13o2(g) ==> 8co2(g) + 10 h2o(l)
in a particular reaction, 5.0 moles of c4h10 are reacted
withan excess of o2. calculate the number of moles of co2
formed.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Asolution at 25 degrees celsius is 1.0 × 10–5 m h3o+. what is the concentration of oh– in this solution?
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Questions

English, 30.10.2019 18:31




Mathematics, 30.10.2019 18:31



History, 30.10.2019 18:31


Health, 30.10.2019 18:31

Mathematics, 30.10.2019 18:31



Mathematics, 30.10.2019 18:31




History, 30.10.2019 18:31


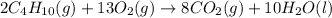
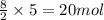 of carbon dioxide.
of carbon dioxide.


