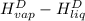Chemistry, 13.09.2019 23:20 SkyeShadow525
The halpy of vaporization of h2o at 1 atm and 100 c is 2259 kj/kg. the heat capacity of liquid water is 4.19 kj/kg. c, and the heat capacity of water vapor is 1.9 kj/kg-c. h20 at 10 bar boils at 179.9 c. what is the enthalpy of vaporization of h20 at 10 bar? you can neglect the effect of pressure. e 2076 kj/kg e 1924 kj/kg e 2259 kj/kg 2442 kj/kg 2594 kj/kg none of the above

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
The halpy of vaporization of h2o at 1 atm and 100 c is 2259 kj/kg. the heat capacity of liquid water...
Questions

English, 30.12.2020 01:00

Computers and Technology, 30.12.2020 01:00

Arts, 30.12.2020 01:00

History, 30.12.2020 01:00




Mathematics, 30.12.2020 01:00



English, 30.12.2020 01:00


Physics, 30.12.2020 01:00

English, 30.12.2020 01:00

Mathematics, 30.12.2020 01:00




Business, 30.12.2020 01:00

Chemistry, 30.12.2020 01:00

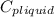 = 4.19
= 4.19 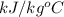
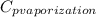 = 1.9
= 1.9 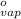 ) at 1 atm and
) at 1 atm and 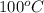 is 2259 kJ/kg
is 2259 kJ/kg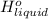 = 0
= 0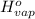 =
= 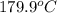 and effect of pressure is not considered. Hence, enthalpy of liquid water at 10 bar and
and effect of pressure is not considered. Hence, enthalpy of liquid water at 10 bar and 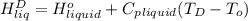
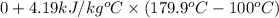
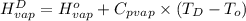
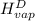 =
= 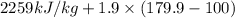
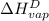 =
=