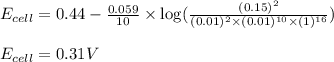Chemistry, 13.09.2019 23:20 michelle230
Calculate the cell potential for a cell operating with the following reaction at 25 degrees celsius, in which [mno4^1-] = .01m, [br^1-] = .01m, [mn^2+] = .15m, and [h^1+] = 1m. the reaction is 2 mno4^1-(aq) + 10 br^1-(aq) + 16 h^1+(aq) --> 2 mn^2+(aq) + 5 br2(l) + 8 h2o(l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
Calculate the cell potential for a cell operating with the following reaction at 25 degrees celsius,...
Questions

Mathematics, 14.10.2019 22:00

Biology, 14.10.2019 22:00



Mathematics, 14.10.2019 22:00




Mathematics, 14.10.2019 22:00




Mathematics, 14.10.2019 22:00



Physics, 14.10.2019 22:00

Mathematics, 14.10.2019 22:00



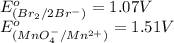
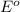 potential will always get reduced and will undergo reduction reaction. Here,
potential will always get reduced and will undergo reduction reaction. Here, 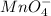 will undergo reduction reaction will get reduced. And, bromine will get oxidized.
will undergo reduction reaction will get reduced. And, bromine will get oxidized.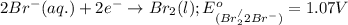 ( × 5)
( × 5)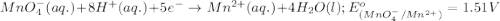 ( × 2)
( × 2)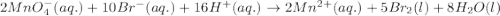
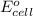 of the reaction, we use the equation:
of the reaction, we use the equation: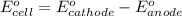
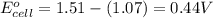
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Mn^{2+}]^2}{[MnO_4^{-}]^2\times [Br^-]^{10}\times [H^+]^{16}}](/tpl/images/0230/4138/86671.png)
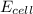 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[H^{+}]=1M](/tpl/images/0230/4138/c7b74.png)
![[Mn^{2+}]=0.15M](/tpl/images/0230/4138/f060a.png)
![[MnO_4^{-}]=0.01M](/tpl/images/0230/4138/b97e7.png)
![[Br^{-}]=0.01M](/tpl/images/0230/4138/bb1d7.png)