

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1


Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
Hydrogen iodide decomposes according to the equation:
2hi(g)h2(g)
+ i2(g),kc = 0.0156 a...
2hi(g)h2(g)
+ i2(g),kc = 0.0156 a...
Questions


Arts, 04.03.2021 01:20

History, 04.03.2021 01:20


Chemistry, 04.03.2021 01:20

Social Studies, 04.03.2021 01:20



Mathematics, 04.03.2021 01:20

Mathematics, 04.03.2021 01:20



Mathematics, 04.03.2021 01:20


Mathematics, 04.03.2021 01:20

History, 04.03.2021 01:20

Mathematics, 04.03.2021 01:20



Chemistry, 04.03.2021 01:20

![\frac{[H_{2}[I_{2} ] }{[HI]^{2}}](/tpl/images/0230/3523/17c92.png) (1)
(1)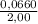 = 0,0330 M
= 0,0330 M![\frac{[x]^2}{[0,0330-2x]^2}](/tpl/images/0230/3523/4723e.png)


