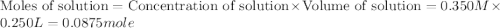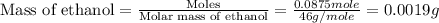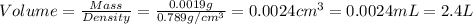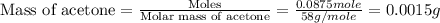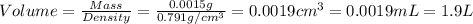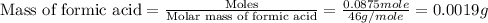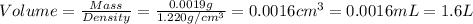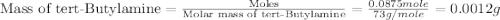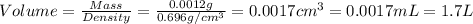Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
Given the densities of the following pure liquids, what volume of each is necessary to make 250 ml o...
Questions

Biology, 31.03.2021 19:40

Mathematics, 31.03.2021 19:40


Geography, 31.03.2021 19:40

Mathematics, 31.03.2021 19:40

Mathematics, 31.03.2021 19:40

Mathematics, 31.03.2021 19:40



Mathematics, 31.03.2021 19:40






Biology, 31.03.2021 19:40

History, 31.03.2021 19:40



History, 31.03.2021 19:40