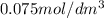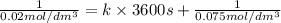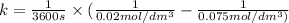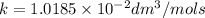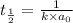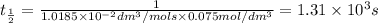Chemistry, 13.09.2019 21:20 haleybug100300
Asecond order reaction of the type a+b> p was carried out in a solution that was initially .075 mol dm-3 in a and .03 mol dm-3 in b. after 1 hour, the concentration of a had fallen to .02 mol dm-3. a. calculate the rate constant. b. what is the half life of the reactant? answers: a. 16.2 dm3mol-hr-, 4.5e-3 dm3mol-s- b. 5.1e3s, 2.1e3 s

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Asecond order reaction of the type a+b> p was carried out in a solution that was initially .075 m...
Questions

Mathematics, 10.12.2021 17:50





Mathematics, 10.12.2021 17:50




Mathematics, 10.12.2021 17:50

Physics, 10.12.2021 17:50

English, 10.12.2021 17:50


Mathematics, 10.12.2021 17:50


Biology, 10.12.2021 17:50

English, 10.12.2021 17:50

Mathematics, 10.12.2021 17:50


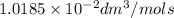 .
. is the half life of the reactant.
is the half life of the reactant.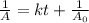
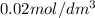
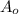 = Initial concentration =
= Initial concentration =