
Chemistry, 13.09.2019 21:20 angel10999
Khoisan salts is the number 1 producer of salts in sa for both local and international markets. 500 kg of kcl is dissolved in sufficient water to make a saturated solution at 370 k. at 370 kthe solubility of kcl is 42 mass %. the solution is cooled to 320 k and the solubility is 31,5 mass % it is assumed that no water is evaporated. 2.1. determine the amount of water is added to the 500 kg of kcl to produce the required saturated solution at 370 k. (3) 2.2. determine the mass of kcl crystals formed after the cooling process to a temperature of 320 k. (use the formula method)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2
You know the right answer?
Khoisan salts is the number 1 producer of salts in sa for both local and international markets. 500...
Questions



Mathematics, 06.07.2019 18:00

Mathematics, 06.07.2019 18:00


Chemistry, 06.07.2019 18:00

Mathematics, 06.07.2019 18:00


History, 06.07.2019 18:00

Physics, 06.07.2019 18:00




Mathematics, 06.07.2019 18:00

Biology, 06.07.2019 18:00

English, 06.07.2019 18:00

History, 06.07.2019 18:00

Physics, 06.07.2019 18:00


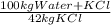 = 1190 kg of water +KCl
= 1190 kg of water +KCl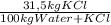 = 375 kg
= 375 kg

