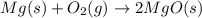When solid magnesium burns, it reacts with oxygen in the air to form a powder called magnesium oxide. a chemist performed this reaction in a lab and found that the mass of the magnesium oxide was greater than the mass of the magnesium. what is the reason behind this increase in mass?
a. the increase in the mass of the magnesium oxide was due to oxygen atoms in the air.
b. the increase in the mass of the magnesium oxide happened because oxygen is heavier than magnesium.
c. the increase in the mass of the magnesium oxide happened because magnesium is heavier than oxygen.
d. the increase in the mass of the magnesium oxide was due to magnesium atoms in the air.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1


You know the right answer?
When solid magnesium burns, it reacts with oxygen in the air to form a powder called magnesium oxide...
Questions

Mathematics, 24.02.2020 06:59


Mathematics, 24.02.2020 06:59







Chemistry, 24.02.2020 07:01




History, 24.02.2020 07:02

Health, 24.02.2020 07:03


Mathematics, 24.02.2020 07:03



Biology, 24.02.2020 07:03