

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
2.4 bromium has two naturally occurring isotopes: 79br, with an atomic weight of 78.918 amu, and 81...
Questions


Chemistry, 17.02.2021 02:50

Mathematics, 17.02.2021 02:50


Computers and Technology, 17.02.2021 02:50

Mathematics, 17.02.2021 02:50


Mathematics, 17.02.2021 02:50



Mathematics, 17.02.2021 02:50

Mathematics, 17.02.2021 02:50



Social Studies, 17.02.2021 02:50

Mathematics, 17.02.2021 02:50


Social Studies, 17.02.2021 02:50


English, 17.02.2021 02:50

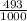 81Br and
81Br and 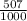 79BR
79BR

