
Chemistry, 12.09.2019 21:30 Benjamincompton07
Atechnician tares a 100.0 ml volumetric flask at 150.00 g. after adding sodium chloride to the flask it then weighs 158.84 g. assuming an error of 0.2 ml in the volumetric volume and 0.005 g in the weight, calculate the molar concentration of sodium chloride and its associated standard deviation.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 23.06.2019 08:50
What happens after sound waves create vibrations in the ear?
Answers: 1

Chemistry, 23.06.2019 09:30
Northern was a learned a fairly cold climate caused by see one from the atlantic ocean, but se was real and tends to be much warmer, sonia look good causes difference. a.cool wins cannot blow across a leg into the south mountains, b.prevent cold air from blowing over into south sea,c.the south is at much higher elevation, so it is closer to the sun, d.suppose early and has a sperience a drastic climate change in the past few years.
Answers: 1
You know the right answer?
Atechnician tares a 100.0 ml volumetric flask at 150.00 g. after adding sodium chloride to the flask...
Questions

Chemistry, 13.02.2020 06:20



Mathematics, 13.02.2020 06:20


Mathematics, 13.02.2020 06:21

Mathematics, 13.02.2020 06:21




Mathematics, 13.02.2020 06:21



Computers and Technology, 13.02.2020 06:21




Engineering, 13.02.2020 06:21

Mathematics, 13.02.2020 06:21

Mathematics, 13.02.2020 06:21

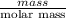
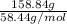
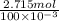 mol/liter
mol/liter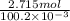 mol/liter
mol/liter 

