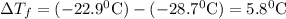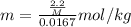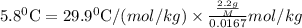Asolution was prepared by dissolving 2.2 g of an unknown solute in 16.7 g of ccl4. a thermal analysis was performed for this solution and it was found that its initial freezing point was – 28.7°c. a reliable source in the bibliography states that for ccl4, t°f = – 22.9°c, and its freezing point lowering constant is kf = 29.9°c/m. calculate the molar mass of the unknown solute.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
Asolution was prepared by dissolving 2.2 g of an unknown solute in 16.7 g of ccl4. a thermal analysi...
Questions










Health, 09.03.2020 23:53



Mathematics, 09.03.2020 23:53







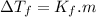
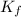 is cryogenoscopic constant of solvent.
is cryogenoscopic constant of solvent.