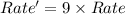Consider the following reaction: 2 no(g) + 2h2(g) → n2(g) + 2 h2o(g) the rate law for this reaction is first order in h2 and second order in no. what would happen to the rate if the initial concentration of no tripled while all other factors stayed the same? the rate will increase by a factor of 9. the rate will decrease by a factor of 3. the rate will double. the rate will triple. the rate will remain constant.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
Consider the following reaction: 2 no(g) + 2h2(g) → n2(g) + 2 h2o(g) the rate law for this reaction...
Questions

Mathematics, 25.11.2020 14:00



Mathematics, 25.11.2020 14:00

English, 25.11.2020 14:00

Mathematics, 25.11.2020 14:00


English, 25.11.2020 14:00

Arts, 25.11.2020 14:00

English, 25.11.2020 14:00


History, 25.11.2020 14:00

Biology, 25.11.2020 14:00

History, 25.11.2020 14:00


Mathematics, 25.11.2020 14:00

Arts, 25.11.2020 14:00

Mathematics, 25.11.2020 14:00

Mathematics, 25.11.2020 14:00

History, 25.11.2020 14:00

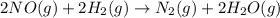
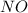 = 2
= 2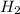 = 1
= 1![Rate=k[NO]^2[H_2]^1](/tpl/images/0229/1480/39530.png)
![Rate'=k[3\times NO]^2[H_2]^1](/tpl/images/0229/1480/e80a2.png)
![Rate'=k[3]^2[NO]^2[H_2]^1](/tpl/images/0229/1480/b88df.png)
![Rate'=k\times 9[NO]^2[H_2]^1](/tpl/images/0229/1480/d2dcb.png)