

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2
You know the right answer?
Consider the following metabolic reaction:
3-phosphoglycerate → 2-phosphoglycerate δg°’ = +4...
3-phosphoglycerate → 2-phosphoglycerate δg°’ = +4...
Questions



English, 17.09.2019 06:30


History, 17.09.2019 06:30

Business, 17.09.2019 06:30

Health, 17.09.2019 06:30

Geography, 17.09.2019 06:30


Mathematics, 17.09.2019 06:30



English, 17.09.2019 06:30

Mathematics, 17.09.2019 06:30




Mathematics, 17.09.2019 06:30

Social Studies, 17.09.2019 06:30

Mathematics, 17.09.2019 06:30

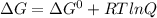
![\Delta G =\Delta G^{0}+RTln\frac{[2-Phosphoglycerate]}{[3-Phosphoglycerate]}](/tpl/images/0229/1031/6e645.png)
![\Delta G = 4.40kJ/mol +0.008314 kJ/mol-K*310Kln\frac{[0.290]}{[2.90]}=-1.53 kJ/mol](/tpl/images/0229/1031/9e71d.png)


