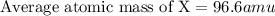An imaginary element (x) on mars is composed of three isotopes, 10.68% of isotope x-95 with a mass of 95.0 amu, 16.90% of isotope x-96 with a mass of 96.0 amu, and 72.42% of isotope x-97 with a mass of 97.0 amu. calculate the atomic mass (in amu) of the element. type in your answer with 3 significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
An imaginary element (x) on mars is composed of three isotopes, 10.68% of isotope x-95 with a mass o...
Questions

Mathematics, 22.07.2019 09:00


Social Studies, 22.07.2019 09:00

Social Studies, 22.07.2019 09:00

History, 22.07.2019 09:00

Mathematics, 22.07.2019 09:00



Arts, 22.07.2019 09:00

History, 22.07.2019 09:00

History, 22.07.2019 09:00




Chemistry, 22.07.2019 09:00



Social Studies, 22.07.2019 09:00


Biology, 22.07.2019 09:00

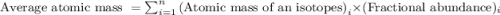 .....(1)
.....(1)![\text{Average atomic mass of X}=[(95\times 0.1068)+(96\times 0.1690)+(97\times 0.7242)]](/tpl/images/0229/1284/93747.png)