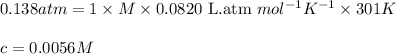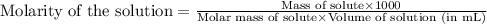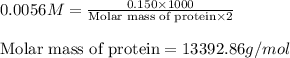Aprotein subunit from an enzyme is part of a research study and needs to be characterized. a total of 0.150 g of this subunit was dissolved in enough water to produce 2.00 ml of solution. at 28 ∘c the osmotic pressure produced by the solution was 0.138 atm. what is the molar mass of the protein?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Aprotein subunit from an enzyme is part of a research study and needs to be characterized. a total o...
Questions


Biology, 06.10.2019 13:00

Geography, 06.10.2019 13:00

Mathematics, 06.10.2019 13:00

Mathematics, 06.10.2019 13:00


Mathematics, 06.10.2019 13:00

Mathematics, 06.10.2019 13:00

Biology, 06.10.2019 13:00


Mathematics, 06.10.2019 13:00

Biology, 06.10.2019 13:00


Mathematics, 06.10.2019 13:00


Mathematics, 06.10.2019 13:00



Mathematics, 06.10.2019 13:00

Computers and Technology, 06.10.2019 13:00

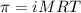
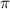 = osmotic pressure of the solution = 0.138 atm
= osmotic pressure of the solution = 0.138 atm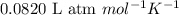
![28^oC=[273+28]=301K](/tpl/images/0229/0989/3c69a.png)