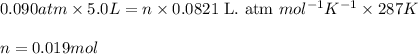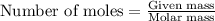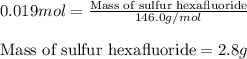Sulfur hexafluoride gas is collected at 14.0°c in an evacuated flask with a measured volume of 5.0l. when all the gas has been collected, the pressure in the flask is measured to be 0.090atm . calculate the mass and number of moles of sulfur hexafluoride gas that were collected. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
Sulfur hexafluoride gas is collected at 14.0°c in an evacuated flask with a measured volume of 5.0l....
Questions

Mathematics, 22.07.2019 10:31

English, 22.07.2019 10:31




Mathematics, 22.07.2019 10:31


Advanced Placement (AP), 22.07.2019 10:31


Biology, 22.07.2019 10:31

Mathematics, 22.07.2019 10:31





Biology, 22.07.2019 10:31




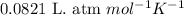
![14^oC=[273+14]K=287K](/tpl/images/0229/0798/930e3.png)