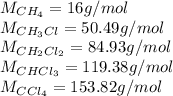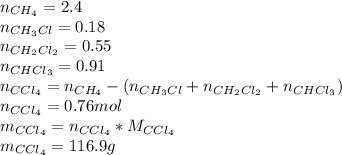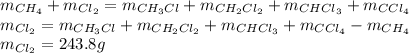Chemistry, 12.09.2019 19:20 tishfaco5000
Methane and chlorine react to form four products: ch3cl, ch2cl2, chcl3, and ccl4. at a particular temperature and pressure, 38.4 g of ch4 was allowed to react with excess cl2 and gave 9.2 g ch3cl, 47.1 g ch2cl2, and 109 g chcl3. all the ch4 reacted. (note: the hydrogen that is displaced from the carbon also combines with cl2 to form hcl.)how many grams of ccl4 were formed? how many grams of cl2 reacted with the ch4?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
Methane and chlorine react to form four products: ch3cl, ch2cl2, chcl3, and ccl4. at a particular t...
Questions


Mathematics, 13.01.2021 23:20

Mathematics, 13.01.2021 23:20

Mathematics, 13.01.2021 23:20

English, 13.01.2021 23:20


History, 13.01.2021 23:20

Mathematics, 13.01.2021 23:20

Biology, 13.01.2021 23:20


Mathematics, 13.01.2021 23:20


Mathematics, 13.01.2021 23:20



Mathematics, 13.01.2021 23:20

English, 13.01.2021 23:20


Social Studies, 13.01.2021 23:20

English, 13.01.2021 23:20