I'm kinda stumped here : /
suppose now that you wanted to determine the density of a small crys...

Chemistry, 12.09.2019 01:30 izzy123abc
I'm kinda stumped here : /
suppose now that you wanted to determine the density of a small crystal to confirm that it is phosphorus. from the literature, you know that phosphorus has a density of 1.82 g/m^3 . how would you prepare 20.0 ml of the liquid mixture having that density from pure samples of chcl^3 ( d= 1.492 g/ml) and chbr^3( d= 2.890 g/ml)? (note: 1 ml = 1 cm^3 .)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2


Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
Questions


History, 02.11.2020 23:20

History, 02.11.2020 23:20

English, 02.11.2020 23:20


Mathematics, 02.11.2020 23:20



Biology, 02.11.2020 23:20







Mathematics, 02.11.2020 23:20

History, 02.11.2020 23:20


Mathematics, 02.11.2020 23:20

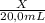 × 1,492 g/mL +
× 1,492 g/mL + 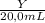 × 2,890 g/mL = 1,82 g/mL (2)
× 2,890 g/mL = 1,82 g/mL (2)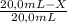 × 2,890 g/mL = 1,82 g/mL
× 2,890 g/mL = 1,82 g/mL 

