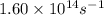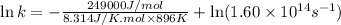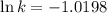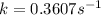Chemistry, 11.09.2019 04:30 shawn20034
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 + hcl the activation energy is 249 kj/mol and the frequency factor is 1.60 × 1014 s−1. find the value of the specific rate constant at 896 k . enter your answer numerically (to 4 decimal places) and in terms of the appropriate units for a first order reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Ethyl chloride vapor decomposes by the first-order reaction c2h5cl → c2h4 + hcl the activation energ...
Questions


Biology, 27.06.2019 05:20



Biology, 27.06.2019 05:20

Mathematics, 27.06.2019 05:20




Chemistry, 27.06.2019 05:20



Mathematics, 27.06.2019 05:20


Social Studies, 27.06.2019 05:20


English, 27.06.2019 05:20



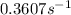
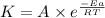
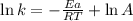 ............(1)
............(1)