
Consider the formation of nitryl fluoride: 2no2(g)+f2(g)⇌2no2f(g) the reaction is first order in f2 and second order overall. what is the rate law? view available hint(s) consider the formation of nitryl fluoride: the reaction is first order in and second order overall. what is the rate law? rate=k[no2]2[f2]2 rate=k[no2][f2]2 rate=k[f2] rate=k[no2] rate=k[no2][f2] rate=k[no2]2[f2]

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
Consider the formation of nitryl fluoride: 2no2(g)+f2(g)⇌2no2f(g) the reaction is first order in f2...
Questions

English, 02.12.2020 20:50





Mathematics, 02.12.2020 20:50




Mathematics, 02.12.2020 20:50




Mathematics, 02.12.2020 20:50


Health, 02.12.2020 20:50


Mathematics, 02.12.2020 20:50


![k[NO_{2}][F_{2}]](/tpl/images/0227/5670/a66e5.png)
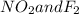 We know overall order of a reaction is summation of individual order with respect to reactants present in rate law equation.Here, overall order of reaction is 2 including first order with respect to
We know overall order of a reaction is summation of individual order with respect to reactants present in rate law equation.Here, overall order of reaction is 2 including first order with respect to 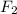 So, rate of reaction should also be first order with respect to another reactant i.e. first order with respect to
So, rate of reaction should also be first order with respect to another reactant i.e. first order with respect to 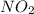 So, rate law: rate =
So, rate law: rate = 

