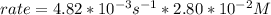Chemistry, 11.09.2019 00:30 nicholasryanencarnac
In carbon tetrachloride proceeds as follows: 2n2o5→4no2+o2. the rate law is first order in n2o5. at 64 ∘c the rate constant is 4.82 ×10−3s−1. part a part complete write the rate law for the reaction. write the rate law for the reaction. rate=2.41×10−3s−1[n2o5] rate=4.82×10−3s−1[n2o5] rate=9.64×10−3s−1[n2o5] rate=4.82×10−3s−1[n2o5]2 previous answers correct part b what is the rate of reaction when [n2o5]= 2.80×10−2 m ?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1
You know the right answer?
In carbon tetrachloride proceeds as follows: 2n2o5→4no2+o2. the rate law is first order in n2o5. at...
Questions

Mathematics, 10.12.2019 02:31

History, 10.12.2019 02:31

Mathematics, 10.12.2019 02:31

English, 10.12.2019 02:31


History, 10.12.2019 02:31



Mathematics, 10.12.2019 02:31


Physics, 10.12.2019 02:31

Mathematics, 10.12.2019 02:31


Biology, 10.12.2019 02:31




Chemistry, 10.12.2019 02:31

Social Studies, 10.12.2019 02:31

Physics, 10.12.2019 02:31

![rate= 4.82*10^{-3}s^{-1} * [N2O5]](/tpl/images/0227/3013/f16e4.png)
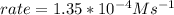
![rate= k * [N2O5]^{x}](/tpl/images/0227/3013/f017a.png)