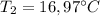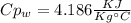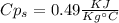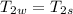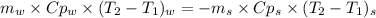Chemistry, 11.09.2019 00:30 sedratkawaiah13
A1.28-kg sample of water at 10.0 °c is in a calorimeter. you drop a piece of steel with a mass of 0.385 kg at 215 °c into it. after the sizzling subsides, what is the final equilibrium temperature? (make the reasonable assumptions that any steam produced condenses into liquid water during the process of equilibration and that the evaporation and condensation don’t affect the outcome, as we’ll see in the next section.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

You know the right answer?
A1.28-kg sample of water at 10.0 °c is in a calorimeter. you drop a piece of steel with a mass of 0....
Questions

History, 22.09.2019 04:30


English, 22.09.2019 04:30


Mathematics, 22.09.2019 04:30




Mathematics, 22.09.2019 04:30


Geography, 22.09.2019 04:30


Computers and Technology, 22.09.2019 04:30



Mathematics, 22.09.2019 04:30