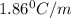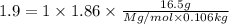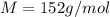Chemistry, 10.09.2019 23:10 blakestuhan
An aqueous solution containing 16.5 g of an unknown molecular (nonelectrolyte) compound in 106.0 g of water was found to have a freezing point of -1.9 ∘c. calculate the molar mass of the unknown compound.

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2


Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2
You know the right answer?
An aqueous solution containing 16.5 g of an unknown molecular (nonelectrolyte) compound in 106.0 g o...
Questions



Health, 13.07.2019 08:20

Biology, 13.07.2019 08:20



Chemistry, 13.07.2019 08:20

English, 13.07.2019 08:20



Mathematics, 13.07.2019 08:20


History, 13.07.2019 08:20


Mathematics, 13.07.2019 08:20





History, 13.07.2019 08:20

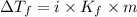
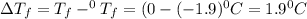 = Depression in freezing point
= Depression in freezing point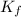 = freezing point constant =
= freezing point constant =